Background: Rigorous monitoring for approximately 4 weeks after chimeric antigen receptor T-cell therapy (CART) is mandated due the potentially fatal toxicity risks of cytokine release syndrome (CRS) and immune effector cell-associated neurologic syndromes (ICANS) during this period, and to allow early appropriate interventions. The US Food and Drug Administration implemented the Risk Evaluation and Mitigation Strategy (REMS) program for approved CART that mandates patients remain within the proximity of the authorized treating center for the monitoring period. The physical and financial burden of stringent monitoring may pose a barrier to CART access, especially for minorities and patients from rural areas and low-income groups. Therefore, evaluating the optimal duration of monitoring and causes of non-relapse mortality (NRM) is an area of unmet need. We evaluated safety outcomes for commercial axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) recipients.
Methods: Patients (pts) ≥ 18 yrs who received an axi-cel or tisa-cel infusion for large B-cell lymphoma between April 2016 and July 2022 at nine academic US medical centers were identified from the Cell Therapy Consortium registry. Descriptive analysis was used to report outcomes. NRM was death due to causes other than disease progression. New-onset CRS and ICANS were reported as a percentage of pts at risk (alive with no prior CRS/ICANS).
Results: A total of 374 pts were included with 216 (58%) receiving axi-cel, and 158 (42%) tisa-cel. Demographics are in Table 1. Median follow-up was 12.4 mo (0.3-58)
Safety: Any grade CRS occurred in 63% pts, more common in axi-cel recipients (80%) compared to tisa-cel (41%), p<0.0001.
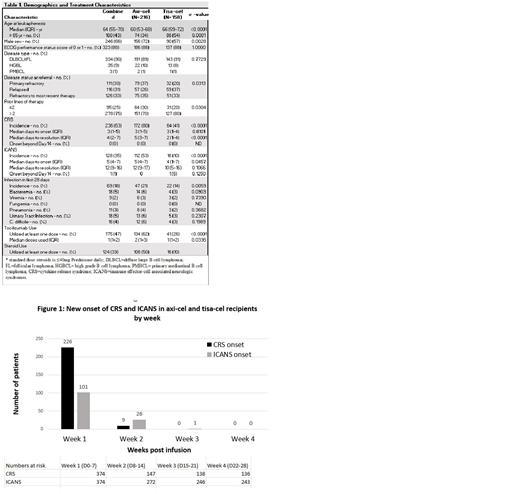
During week 1 (Day 0-7), new-onset CRS occurred in 60.4% of the total population including 75.4% of axi-cel and 39.8% of tisa-cel recipients. New CRS onset in week 2 (Day 8-14) was seen in 6.1% of pts (15.4% axi-cel vs 1% tisa-cel). There was no new onset CRS in Week 3 or beyond. The median time to CRS onset was 3 days (0-13). (Figure 1)
Unresolved CRS beyond Day 14 occurred in 7.2% (8.7% of axi-cel vs 3.1% of tisa-cel recipients), of which only 1.7% of pts (all axi-cel recipients) had unresolved CRS beyond Day 28. The median time to resolution was 4 days (IQR 25-75%: 2-7). 98.3% of CRS resolved by Day 28.
Any grade ICANS was seen in 35% of pts, more common in axi-cel (52%) compared to tisa-cel (10%), p<0.0001.
During Week 1, new-onset ICANS was seen in 27% of of the total population including 40.7% of axi-cel and 8.2% of tisa-cel recipients. New ICANS in week 2 was seen in 9.5% of pts (18.8% of axi-cel vs 2.8% of tisa-cel recipients). During week 3, new-onset ICANS occurred in only 1 tisa-cel recipient (0.4%). There were no new cases in week 4 and beyond. The median time to ICANS onset was 5 days (1-21).
Unresolved or ongoing ICANS beyond Day 14 occurred in 32.8% of pts (34.8% of axi-cel: vs 18.7% of tisa-cel recipients), and beyond Day 28 in 7% of pts (7.1% of axi-cel vs 6.3% of tisa-cel recipients). The median time to ICANS resolution was 12 days (IQR 25-75%: 9-16). 93% of ICANS resolved by Day 28.
Toxicity Management: Tocilizumab was utilized in 47% of pts (axi-cel 62% vs tisa-cel 26%) and 33% of pts received steroids (axi-cel 50% vs 10% tisa-cel) for management of CART-related toxicities.
Other Complications: Infectious complications from Day 0-28 were seen in 18.5% of the total population and more commonly in axi-cel (22%) compared to tisa-cel (13.9%) recipients, p=0.006. Grade ≥4 neutropenia from Day 0-28 days was seen in 66% of the total population (81% axi-cel vs 46% tisa-cel). 82% of the total population did not have Grade ≥4 neutropenia beyond Week 2, and 93% had no Grade ≥4 neutropenia beyond Week 4.
NRM: Day 0-28 NRM included 4 deaths (3 related to ICANS and 1 infection), and Day 29-90 NRM included 7 deaths (3 infections, 1 ICANS, 1 cardiac, 2 other).
Conclusions: This large multicenter retrospective analysis evaluates patterns of CRS, ICANS, and NRM in recipients of axi-cel and tisa-cel. While NRM is mainly due to infections and ICANS, our study suggests that beyond 2 weeks following CART infusion, the incidence of new onset CRS and ICANS is low. This data supports further investigation into individualized monitoring strategies for stable patients. A flexible monitoring period may help to decrease the financial and physical burden for patients and make CART more accessible and feasible, especially for those with limited socioeconomic support.
Disclosures
Porter:Tmunity: Patents & Royalties; Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Sana Therapeutics: Consultancy, Current equity holder in publicly-traded company; Wiley and Sons Publishing: Honoraria; Mirror Biologics: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Genentech: Current equity holder in publicly-traded company; DeCart: Membership on an entity's Board of Directors or advisory committees; Capstan Bio: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Angiocrine Bio: Membership on an entity's Board of Directors or advisory committees. Bachanova:ADC: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Other: DSMB; Citius: Research Funding; BMS: Research Funding; Gamida Cell: Research Funding; Incyte: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Nastoupil:Regeneron: Honoraria; AstraZeneca: Honoraria; Gilead Sciences/Kite Pharma: Honoraria, Research Funding; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria; AbbVie: Honoraria. Riedell:Karyopharm Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sana Biotechnology: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nektar Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; MorphoSys: Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Pharmacyclics: Consultancy; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nkarta: Research Funding; Genmab: Consultancy; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Research Funding; CRISPR Therapeutics: Research Funding; Xencor: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Research Funding; Calibr: Research Funding; CVS Caremark: Consultancy; Celgene/ Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Perales:Vor Biopharma: Consultancy, Honoraria; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; MorphoSys: Consultancy, Honoraria; Sellas Life Sciences: Consultancy; Caribou: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Research Funding; NexImmune: Consultancy, Current equity holder in publicly-traded company; Takeda: Consultancy, Honoraria; VectivBio AG: Consultancy, Honoraria; Syncopation: Honoraria; Cidara Therapeutics: Consultancy, Other; Allovir: Consultancy; Allogene: Research Funding; Merck: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; DSMB: Other; Adicet: Honoraria; Miltenyi Biotec: Honoraria; Astellas: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; Servier: Other; Medigene: Consultancy, Other; AbbVie: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; Celgene: Honoraria; Kite: Consultancy, Honoraria, Research Funding; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Miltenyi Biotec: Consultancy, Honoraria, Research Funding. Maziarz:Kite: Consultancy; Orca Therapeutics: Research Funding; Athersys: Other: Patent holder; Gamida: Research Funding; AlloVir: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Shah:Janssen: Research Funding; ArcellX: Other: DSMB; Beyond Spring: Research Funding; Amgen: Research Funding; BMS: Research Funding. Chen:Fate: Research Funding; Intellia: Consultancy; Kite: Consultancy, Research Funding; Elsevier: Consultancy; Novartis: Research Funding. Oluwole:Pfizer: Consultancy, Honoraria, Research Funding; ADC: Consultancy, Speakers Bureau; TGR: Consultancy; Daiichi Sankyo: Research Funding; Epizyme: Consultancy; Novartis: Consultancy; Nektar: Consultancy; Kite, a Gilead Company/ Gilead: Consultancy, Research Funding; Cargo: Consultancy; Caribou: Consultancy; AbbVie: Consultancy; Gilead: Consultancy, Honoraria; Allogene: Research Funding. Bishop:Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau; Tmunity: Research Funding; Sana Biotechnology: Consultancy; Chimeric Therapeutics: Consultancy; Iovance: Consultancy; WindMIL Therapeutics: Consultancy; Triumvira: Research Funding; Agios: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; CRISPR Therapeutics: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel support, Research Funding; Bluebird Bio: Consultancy; Arcellx: Consultancy, Research Funding; Immatics: Research Funding; Autolus: Consultancy, Research Funding; BMS, Kite/Gilead, Servier, AstraZeneca, ADC Therapeutics, Incyte: Speakers Bureau; ADC Therapeutics: Speakers Bureau; KITE/Gilead, Novartis, CRISPR Therapeutics, Autolus Therapeutics, BMS/JUNO Therapeutics, Incyte, Sana Biotechnology, Iovance Biotherapeutics, In8bio, Chimeric Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Speakers Bureau; BMS: Honoraria, Other: Travel support, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Incyte: Honoraria, Other: Travel support, Speakers Bureau; Celgene: Honoraria. McGuirk:Allovir: Consultancy, Research Funding; Juno Therapeutics: Consultancy; Pluristem Therapeutics: Research Funding; Magenta Therapeutics: Consultancy; Bellicum Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; Fresenius Biotech: Research Funding; Novartis: Research Funding; EcoR1 Capital: Consultancy; Kite: Consultancy, Research Funding; Gamida Cell: Research Funding. Ahmed:Kite/Gilead: Consultancy, Research Funding; BMS: Other: Ad Board.